Octane: what it actually means and does
#1
Thread Starter
The Black Pearl
iTrader: (2)
Joined: Jan 2009
Posts: 212
Likes: 0
From: Houston, Texas
Octane: what it actually means and does
I have been trolling the site lately reading over threads to try and educate myself about the nuances of the 8. Although there are some really good definitions and explanations of some things in here, there is some serious misinformation about others. One of the things that I know a fair deal about due to my occupations is energy, specifically petroleum and natural gas. While reading a thread about gasoline, there were some posts about octane that I found to be fairly misleading and some to be complete nonsense. So, in an effort to try and clear up what octane actually is and how it relates to use in fuel, I am writing this educational piece. Please refrain from calling me an idiot or that I don't know what I am talking about, as I doubt you can prove that you know more on this subject than I, with the exception of a few members on this site who have actual proven track results based on fuel use, consumption and power levels. Much love to them.
So what is Octane?
Octane is the measure of the resistance of a fuel, specifically in this case gasoline/petrol, to spontaneous auto-ignition due to various conditions in the engine; such as heat and compression
The rating that you see is a laboratory measured value of resistance in a test engine. This test fuel is defined by its comparison to a mixture fuel of iso-octane and heptane (n-heptane). By comparing these fuels, the resistance measure in the test fuel is compared to determine what mixture has of octane and heptane is most closely similar. The reason this is done is due to what was originally the 100-octane scale, where iso-octane has a value of 100 and heptane a value of 0. So, a fuel with an octane rating of 90 would be a mixture composed of 90% iso-octane and 10% heptane. This does not mean that the fuel being tested is composed of iso-octane and heptane, but has the same resistance properties of said mixture. However, due to the advent of fuels that have better knock-resistance that iso-octane, octane ratings above 100 are now common.
Octane ratings are not a relation to the amount of energy content in a fuel.
It is only the rating of the fuels ability to burn in a controlled manner, or its resistance to detonation or explosion in an uncontrolled manner.
Another reason why there is some misunderstanding about octane levels and how they relate to vehicles is the disconnection from how the US and Canada rate octane levels compared to how Europe, Asia and Australia rate their octane levels.
3 different rating systems
1.) RON - Research Octane Number - This is the rating number that is associated with the explanation given above. The fuel is tested against the known fuel mixtures of iso-octane and heptane. The test engine is run under variable compressions as well to give a more rounded number.
2.) MON - Motor Octane Number - sometimes called the aviation lean octane rating - This is a more reflective measure of a fuels behavior as it is done under a load of around 900 RPM instead of the 600 RPM of the RON. The differences in the test include: different but similar engines, a preheated fuel mixture, increased engine speeds, and most importantly, a variable valve timing sequence to stress test the fuels anti-knock resistance. The average MON number can range from 5 to 9 or 10 points lower than the results of a RON test.
3.) AKI - Anti-Knock Index - This is the system that is used in the US and Canada. This value is the average between the RON and MON of a fuel. Other names for this system include: Road Octane Number (RdON) and the Pump Octane Number (PON). This is why on the octane sticker of a fueling station pump, the octane sticker has the formula R + M / 2. This is the RON + MON / 2 formula which equals the average of the RON and MON ratings.
Because of these different ratings, the octane rating that our friends across the pond state that they are using will almost always be higher by 4-5 points than the numbers that we are giving.
Some ratings for fuels giving the RON, MON and AKI numbers. These can be found on the IUPAC website and the Purdue chemical database. I will post the links at the bottom of the post.
Fuel, RON, MON, AKI
Baseline Fuels
n-Heptane, 0, 0, 0
iso-Octane, 100, 100, 100
Common Fuels
Reg unleaded (US), 91-92, 82-83, 87
Super, "EuroSuper", 95, 85-86, 90-91
Premium (US), 97-98, 88-89, 93
High-Octane/Race Fuel/alternative
BP "Ultimate" 102, 102, 93-94, 97-98
Propane, 110, couldn't find, NA
E85, NA, NA, 100-105*
Ethanol, 129, 102, 116*
Methanol, 133, 105, 119*
Methane (CNG), 135, 122, 129
Thought I should include this having seen it somewhere
Hydrogen, >130, <0**, NA
* There is still some debate throughout the forums as to the effect on the rotary engines of using an alcohol based fuel due to the effect of the OH- group released in combustion and the effect that group has on the integrity of the seals within the engine. As for me, I feel that as a known free radical and oxidizer, anything that is introducing these compounds into a highly controlled environment is potentially damaging. I am not saying that it is, but that it could be. One of those situations where someone will have to stress test the engine comparing alcohol and non-alcohol based fuels in simpatico and then pull the engines apart and observe the effects.
** Hydrogen is interesting in that it has a VERY low ignition energy and incredibly high combustion and ignition rate. This is not the best for an engine, as much of the energy will be lost to heat, which will cause a severe loss in power when acting upon a piston or rotor. Cyclic style engines do not perform well due to rotational inertia when using hydrogen as the flame speed burns to quickly to enact enough force on the crank to be efficient. However, in nozzle propulsion, the quick flame and low activation energy is preferred as the quick flame ignition allows for directional motion without resistance to energy flow except for in the opposite direction which allows full energy transfer to the source of fuel. This is seen in rocket propulsion in which the forced ejection of fuel through a nozzle allows the energy to transfer directly to the fuselage or rocket body without counter-force from the outside of the nozzle.
So why do we care about the octane rating?
So, after a long drawn out explanation, we care about octane because it dictates the activation energy of a combustion reaction. Higher octane = more energy required to cause ignition. Higher octane also = less of a chance that a higher compression will force detonation/auto-ignition.
Because of this, in more powerful engines, a higher octane is preferred. Since high output, finely tuned engines rely on the minute accuracy of compression vs. air/fuel vs. timing of ignition; the reduction in detonation allows the engine to operate at higher tuned levels. When detonation occurs, the combustion energy will be placed upon the piston/rotor before it is in the correct position to allow for maximum energy transfer to the crankshaft. This is what is occurring when you hear that ping noise on an interference style engine. Should detonation occur, the knock sensor that is common in most vehicles will detect the knock and send a signal to the engine/ECU to retard the timing to prevent the knock from occurring. This will in turn reduce the output of the engine. This is less of a problem when the throttle is in a partially opened position, as the pressure on the manifold will still be below atmospheric and only a portion of the total engine power is available. However, once the throttle is WOT, the pressure within the manifold is brought to atmospheric pressure and even higher in forced induction applications. This increase in compression leads to an increase in the activation energy that is present and the potential for auto-ignition is greater. By using a higher-octane fuel, you can reduce the potential for auto-ignition by increasing the energy requirement for auto-ignition to counter the energy current in the engine.
So, while higher-octane fuels can reduce the activation energy, it does NOT increase the amount of energy stored in the fuel. An engine can only produce so much energy out of a fuel based on the density of the energy contained within it. Raising the octane does not increase the energy; it only increases the amount of energy need from the engine to ignite it. So, without adding more hydrocarbon content to the fuel, you cannot increase the power you get from it. Different octane rating does not translate to different energy density.
IMPORTANT
While using a higher-octane fuel does not harm your engine, using a lower octane fuel than you car was designed for can cause severe damage to it. Should auto-ignition occur, and the knock sensor, or lack thereof, fails to function correctly, the counter-force on the placed upon the piston/rotors and the crank will begin over time to stress and eventually warp and disfigure them. While it can be fixed, the damage to these parts can be known to affect other parts of the engine including housings as well as counter forcing the other pistons and rotors forcing them out of balance and screwing with the timing. While not as scary on a rotary, the threat to interference style engines can be devastating. Not only like bent valves and springs, but also having retainers wrapped up around a camshaft. *Raises hand for being guilty of this on a 98 eclipse*.
History:
Russell Marker devised the scale while working for the Ethyl Corporation around the mid 1920's. He used n-heptane simply because it was the most readily available and pure compound that was available to him at the time.
Current fuels:
While fuels in Europe and Asia below the 91 RON are very uncommon, it is still possible to purchase 80 RON (76 MON) fuel in Russia. *Please don't use this in your 8... unless you hate it*
In the Rocky Mountains it is possible to still get 85 AKI fuel due to the elevation reducing overall compression which in turn deters knock. 87 AKI at sea level is approx. to 85 at 5-7k ft.
In the US Midwest, E85 is commonly available with an AKI of 105. The tuning efficiency of this fuel can be seen by the forced induction tuning done by HYMEE (click here) while testing the power outputs of his SC setup. The higher octane allowed him to more finely tune the ignition, which in turn allowed more power. Not due to the fuel, but due to the more precise tuning.
EDIT/ADDITION part 1
I have been getting alot of messages regarding the relationship between octane and flame speed. So much so that I have decided to address them publically in the thread.
I will start by saying that Octane and flame speed are virtually unrelated.
Octane as explained above is the resistance of a fuel to auto-ignition.
Flame-speed is a stoichemetric reaction rate or a fuel kinetics rate which relates to the speed in which the hydrogens and oxygens of a fuel can be stripped away by the the combustion reaction. The speed of flame for a fuel will remain the same, variations can occur, unless the hydrocarbon content of the fuel is dramatically changed. Additives such as octane do not fundamentally change the content of the fuel, and therefore do not change the flame speed. There are fuels such as hydrogen gas which consist of only H-H bonds that can be broken quickly and do not require oxidation of the H-H bond in order to break. In this case, the flame speed would be incredibly fast. For other fuels such as gasoline with chemical structures ranging from C6H14 all the way to C12H26, average of C8H18, the time, while miniscule in nature, to strip the Hydrogens from the carbon backbone and allow reaction with atmospheric O2 is kinetically much longer. Remember that energy from any fuel is just the energy released when a chemical or nuclear bond is disrupted or broken. So, unless you are able to break the bonds faster, or make them fundamentally easier to break, you will not increase flame speed or power.
*fun fact* There is some correlation in lower BTU fuels like LH2 and LOx, that require more fuel for = power. Think of the space shuttle. The solid booster rockets' and the SLWT ET's fuel pumps can drain an olympic sized swimming pool in 60 seconds. That is alot of fuel output. Bootstrap space travel at its finest.
For most people, flame speed is not a concern. However, in high output racing engines, flame speed is one of the most important tuning aspects. At low RPM, flame speed is negligible because the reaction is usually allowed to complete before the next cycle begins. But under high RPM where the spark plugs may fire as much as 120-150 times per second, the flame speed is paramount. Racing engines require that the fuel be able to burn as completely as possible and exert as much force on the crank very quickly before the cycle begins again. Unspent fuel due to a slower flame speed = loss of power.
So, after all of this, I can answer for you another question. Does octane of a higher number burn slower? Answer. possibly, but the difference in flame speed is so minute due to the slight change in addititves, that it would be completely unnoticible and would not affect any of our engines. The amount of octane that is included in the fuel is not enough to significantly alter the hydrocarbon content, which in turn means it does not change the power or the flame speed.
Edit/Addition 2: Chemistry of n-heptane and 2,2,4-trimethylpentane (iso-octane or octane)
Woo Hoo, Chemistry lesson time:
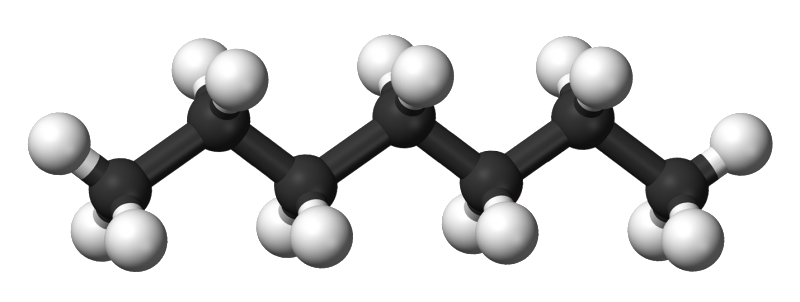
Heptane, or n-heptane is a basic hydrocarbon chain like a stick with nodes on it. Heptane is C-C-C-C-C-C-C with hydrogen filling the valence electron pairings on the Carbons so it looks more like CH3-CH2-CH2-CH2-CH2-CH2-CH3.
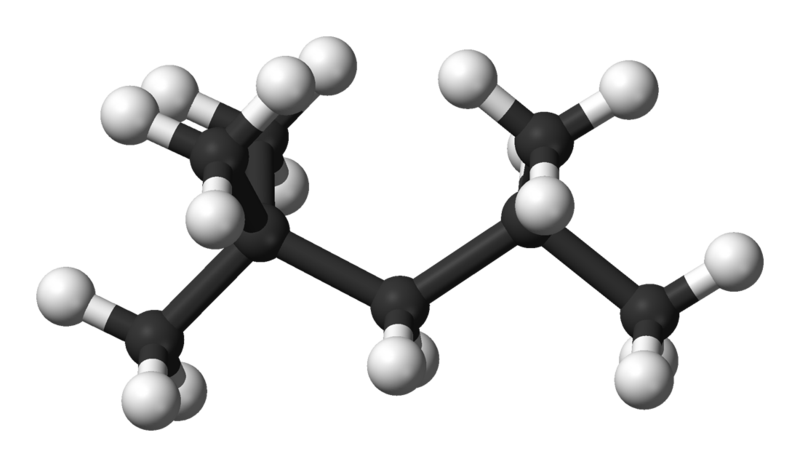
whereas octane (or iso-octane) is actually 2,2,4-trimethylpentane which looks like a stick with smaller sticks coming off of it (pardon the elementary description). Iso-octane has that same Carbon backbone style structure but with only 5 carbons, but, it contains 3 extra Carbon groups attached to the 2nd (2 methyl groups) and 4th (single methyl group) primary carbons so it looks more like CH3-C(CH3)2-CH2-CH(CH3)-CH3.
Essentially:
The empirical formula for iso-octane contains the average of C8H18 for gasoline. So it really is having no affect on total energy content. N-heptane is C7H16 so it is actually below the average. The difference of only 1 total hydrogen is not significant enough to change the content of fuel, but requires less energy to break down its carbon structure. Octane on the other hand has more C-C bonds with more hydrogen. Due to the angles of the C-C bonds on the 2nd and 4th primary carbons, more energy is required to break down the chain.
This energy requirement is why higher [octane] ultimately reduces auto-ignition chances as more energy is required to cause combustion. Not because of more hydrogen, but because of the geometry of the bond angles and its "iso" structure
Addition 3: differences in grade
Something was brought up and I wanted to address it. In typical reformulated and conventional unleaded gasolines, the way the different grades are determined is by the concentration of iso-octane within the gasoline. 87 AKI will contain less iso-octane than 91 AKI. From what I have been told and found in research, this is the way it is done. Racing fuels and E85 type fuels are done slightly differently I believe. If I am wrong please correct me. From speaking with some of my colleagues, the way it is done is by drawing fuels from multiple USTs that are on site at the fueling station. The higher grade fuel simply pulls from the higher octane tank for a longer period of time thereby raising the octane rating of the pumped fuel. I am putting this in here for forum verification. Not to disavow my colleagues, but to see if anyone else has heard this as well.
Links used as references:
IUPAC database
Purdue Petroleum and Coal Publications
Hydrogen Fuel
E85 info
CNG info
Hope this helps. If you would like to add anything that I have missed, please let me know. Also, if the statement you wish to add is egregious, please provide a source for it. I do not want this to turn into someone’s opinion about how they think it works. Thanks.
So what is Octane?
Octane is the measure of the resistance of a fuel, specifically in this case gasoline/petrol, to spontaneous auto-ignition due to various conditions in the engine; such as heat and compression
The rating that you see is a laboratory measured value of resistance in a test engine. This test fuel is defined by its comparison to a mixture fuel of iso-octane and heptane (n-heptane). By comparing these fuels, the resistance measure in the test fuel is compared to determine what mixture has of octane and heptane is most closely similar. The reason this is done is due to what was originally the 100-octane scale, where iso-octane has a value of 100 and heptane a value of 0. So, a fuel with an octane rating of 90 would be a mixture composed of 90% iso-octane and 10% heptane. This does not mean that the fuel being tested is composed of iso-octane and heptane, but has the same resistance properties of said mixture. However, due to the advent of fuels that have better knock-resistance that iso-octane, octane ratings above 100 are now common.
Octane ratings are not a relation to the amount of energy content in a fuel.
It is only the rating of the fuels ability to burn in a controlled manner, or its resistance to detonation or explosion in an uncontrolled manner.
Another reason why there is some misunderstanding about octane levels and how they relate to vehicles is the disconnection from how the US and Canada rate octane levels compared to how Europe, Asia and Australia rate their octane levels.
3 different rating systems
1.) RON - Research Octane Number - This is the rating number that is associated with the explanation given above. The fuel is tested against the known fuel mixtures of iso-octane and heptane. The test engine is run under variable compressions as well to give a more rounded number.
2.) MON - Motor Octane Number - sometimes called the aviation lean octane rating - This is a more reflective measure of a fuels behavior as it is done under a load of around 900 RPM instead of the 600 RPM of the RON. The differences in the test include: different but similar engines, a preheated fuel mixture, increased engine speeds, and most importantly, a variable valve timing sequence to stress test the fuels anti-knock resistance. The average MON number can range from 5 to 9 or 10 points lower than the results of a RON test.
3.) AKI - Anti-Knock Index - This is the system that is used in the US and Canada. This value is the average between the RON and MON of a fuel. Other names for this system include: Road Octane Number (RdON) and the Pump Octane Number (PON). This is why on the octane sticker of a fueling station pump, the octane sticker has the formula R + M / 2. This is the RON + MON / 2 formula which equals the average of the RON and MON ratings.
Because of these different ratings, the octane rating that our friends across the pond state that they are using will almost always be higher by 4-5 points than the numbers that we are giving.
Some ratings for fuels giving the RON, MON and AKI numbers. These can be found on the IUPAC website and the Purdue chemical database. I will post the links at the bottom of the post.
Fuel, RON, MON, AKI
Baseline Fuels
n-Heptane, 0, 0, 0
iso-Octane, 100, 100, 100
Common Fuels
Reg unleaded (US), 91-92, 82-83, 87
Super, "EuroSuper", 95, 85-86, 90-91
Premium (US), 97-98, 88-89, 93
High-Octane/Race Fuel/alternative
BP "Ultimate" 102, 102, 93-94, 97-98
Propane, 110, couldn't find, NA
E85, NA, NA, 100-105*
Ethanol, 129, 102, 116*
Methanol, 133, 105, 119*
Methane (CNG), 135, 122, 129
Thought I should include this having seen it somewhere
Hydrogen, >130, <0**, NA
* There is still some debate throughout the forums as to the effect on the rotary engines of using an alcohol based fuel due to the effect of the OH- group released in combustion and the effect that group has on the integrity of the seals within the engine. As for me, I feel that as a known free radical and oxidizer, anything that is introducing these compounds into a highly controlled environment is potentially damaging. I am not saying that it is, but that it could be. One of those situations where someone will have to stress test the engine comparing alcohol and non-alcohol based fuels in simpatico and then pull the engines apart and observe the effects.
** Hydrogen is interesting in that it has a VERY low ignition energy and incredibly high combustion and ignition rate. This is not the best for an engine, as much of the energy will be lost to heat, which will cause a severe loss in power when acting upon a piston or rotor. Cyclic style engines do not perform well due to rotational inertia when using hydrogen as the flame speed burns to quickly to enact enough force on the crank to be efficient. However, in nozzle propulsion, the quick flame and low activation energy is preferred as the quick flame ignition allows for directional motion without resistance to energy flow except for in the opposite direction which allows full energy transfer to the source of fuel. This is seen in rocket propulsion in which the forced ejection of fuel through a nozzle allows the energy to transfer directly to the fuselage or rocket body without counter-force from the outside of the nozzle.
So why do we care about the octane rating?
So, after a long drawn out explanation, we care about octane because it dictates the activation energy of a combustion reaction. Higher octane = more energy required to cause ignition. Higher octane also = less of a chance that a higher compression will force detonation/auto-ignition.
Because of this, in more powerful engines, a higher octane is preferred. Since high output, finely tuned engines rely on the minute accuracy of compression vs. air/fuel vs. timing of ignition; the reduction in detonation allows the engine to operate at higher tuned levels. When detonation occurs, the combustion energy will be placed upon the piston/rotor before it is in the correct position to allow for maximum energy transfer to the crankshaft. This is what is occurring when you hear that ping noise on an interference style engine. Should detonation occur, the knock sensor that is common in most vehicles will detect the knock and send a signal to the engine/ECU to retard the timing to prevent the knock from occurring. This will in turn reduce the output of the engine. This is less of a problem when the throttle is in a partially opened position, as the pressure on the manifold will still be below atmospheric and only a portion of the total engine power is available. However, once the throttle is WOT, the pressure within the manifold is brought to atmospheric pressure and even higher in forced induction applications. This increase in compression leads to an increase in the activation energy that is present and the potential for auto-ignition is greater. By using a higher-octane fuel, you can reduce the potential for auto-ignition by increasing the energy requirement for auto-ignition to counter the energy current in the engine.
So, while higher-octane fuels can reduce the activation energy, it does NOT increase the amount of energy stored in the fuel. An engine can only produce so much energy out of a fuel based on the density of the energy contained within it. Raising the octane does not increase the energy; it only increases the amount of energy need from the engine to ignite it. So, without adding more hydrocarbon content to the fuel, you cannot increase the power you get from it. Different octane rating does not translate to different energy density.
IMPORTANT
While using a higher-octane fuel does not harm your engine, using a lower octane fuel than you car was designed for can cause severe damage to it. Should auto-ignition occur, and the knock sensor, or lack thereof, fails to function correctly, the counter-force on the placed upon the piston/rotors and the crank will begin over time to stress and eventually warp and disfigure them. While it can be fixed, the damage to these parts can be known to affect other parts of the engine including housings as well as counter forcing the other pistons and rotors forcing them out of balance and screwing with the timing. While not as scary on a rotary, the threat to interference style engines can be devastating. Not only like bent valves and springs, but also having retainers wrapped up around a camshaft. *Raises hand for being guilty of this on a 98 eclipse*.
History:
Russell Marker devised the scale while working for the Ethyl Corporation around the mid 1920's. He used n-heptane simply because it was the most readily available and pure compound that was available to him at the time.
Current fuels:
While fuels in Europe and Asia below the 91 RON are very uncommon, it is still possible to purchase 80 RON (76 MON) fuel in Russia. *Please don't use this in your 8... unless you hate it*
In the Rocky Mountains it is possible to still get 85 AKI fuel due to the elevation reducing overall compression which in turn deters knock. 87 AKI at sea level is approx. to 85 at 5-7k ft.
In the US Midwest, E85 is commonly available with an AKI of 105. The tuning efficiency of this fuel can be seen by the forced induction tuning done by HYMEE (click here) while testing the power outputs of his SC setup. The higher octane allowed him to more finely tune the ignition, which in turn allowed more power. Not due to the fuel, but due to the more precise tuning.
EDIT/ADDITION part 1
I have been getting alot of messages regarding the relationship between octane and flame speed. So much so that I have decided to address them publically in the thread.
I will start by saying that Octane and flame speed are virtually unrelated.
Octane as explained above is the resistance of a fuel to auto-ignition.
Flame-speed is a stoichemetric reaction rate or a fuel kinetics rate which relates to the speed in which the hydrogens and oxygens of a fuel can be stripped away by the the combustion reaction. The speed of flame for a fuel will remain the same, variations can occur, unless the hydrocarbon content of the fuel is dramatically changed. Additives such as octane do not fundamentally change the content of the fuel, and therefore do not change the flame speed. There are fuels such as hydrogen gas which consist of only H-H bonds that can be broken quickly and do not require oxidation of the H-H bond in order to break. In this case, the flame speed would be incredibly fast. For other fuels such as gasoline with chemical structures ranging from C6H14 all the way to C12H26, average of C8H18, the time, while miniscule in nature, to strip the Hydrogens from the carbon backbone and allow reaction with atmospheric O2 is kinetically much longer. Remember that energy from any fuel is just the energy released when a chemical or nuclear bond is disrupted or broken. So, unless you are able to break the bonds faster, or make them fundamentally easier to break, you will not increase flame speed or power.
*fun fact* There is some correlation in lower BTU fuels like LH2 and LOx, that require more fuel for = power. Think of the space shuttle. The solid booster rockets' and the SLWT ET's fuel pumps can drain an olympic sized swimming pool in 60 seconds. That is alot of fuel output. Bootstrap space travel at its finest.
For most people, flame speed is not a concern. However, in high output racing engines, flame speed is one of the most important tuning aspects. At low RPM, flame speed is negligible because the reaction is usually allowed to complete before the next cycle begins. But under high RPM where the spark plugs may fire as much as 120-150 times per second, the flame speed is paramount. Racing engines require that the fuel be able to burn as completely as possible and exert as much force on the crank very quickly before the cycle begins again. Unspent fuel due to a slower flame speed = loss of power.
So, after all of this, I can answer for you another question. Does octane of a higher number burn slower? Answer. possibly, but the difference in flame speed is so minute due to the slight change in addititves, that it would be completely unnoticible and would not affect any of our engines. The amount of octane that is included in the fuel is not enough to significantly alter the hydrocarbon content, which in turn means it does not change the power or the flame speed.
Edit/Addition 2: Chemistry of n-heptane and 2,2,4-trimethylpentane (iso-octane or octane)
Woo Hoo, Chemistry lesson time:
Heptane, or n-heptane is a basic hydrocarbon chain like a stick with nodes on it. Heptane is C-C-C-C-C-C-C with hydrogen filling the valence electron pairings on the Carbons so it looks more like CH3-CH2-CH2-CH2-CH2-CH2-CH3.

whereas octane (or iso-octane) is actually 2,2,4-trimethylpentane which looks like a stick with smaller sticks coming off of it (pardon the elementary description). Iso-octane has that same Carbon backbone style structure but with only 5 carbons, but, it contains 3 extra Carbon groups attached to the 2nd (2 methyl groups) and 4th (single methyl group) primary carbons so it looks more like CH3-C(CH3)2-CH2-CH(CH3)-CH3.

Essentially:
The empirical formula for iso-octane contains the average of C8H18 for gasoline. So it really is having no affect on total energy content. N-heptane is C7H16 so it is actually below the average. The difference of only 1 total hydrogen is not significant enough to change the content of fuel, but requires less energy to break down its carbon structure. Octane on the other hand has more C-C bonds with more hydrogen. Due to the angles of the C-C bonds on the 2nd and 4th primary carbons, more energy is required to break down the chain.
This energy requirement is why higher [octane] ultimately reduces auto-ignition chances as more energy is required to cause combustion. Not because of more hydrogen, but because of the geometry of the bond angles and its "iso" structure
Addition 3: differences in grade
Something was brought up and I wanted to address it. In typical reformulated and conventional unleaded gasolines, the way the different grades are determined is by the concentration of iso-octane within the gasoline. 87 AKI will contain less iso-octane than 91 AKI. From what I have been told and found in research, this is the way it is done. Racing fuels and E85 type fuels are done slightly differently I believe. If I am wrong please correct me. From speaking with some of my colleagues, the way it is done is by drawing fuels from multiple USTs that are on site at the fueling station. The higher grade fuel simply pulls from the higher octane tank for a longer period of time thereby raising the octane rating of the pumped fuel. I am putting this in here for forum verification. Not to disavow my colleagues, but to see if anyone else has heard this as well.
Links used as references:
IUPAC database
Purdue Petroleum and Coal Publications
Hydrogen Fuel
E85 info
CNG info
Hope this helps. If you would like to add anything that I have missed, please let me know. Also, if the statement you wish to add is egregious, please provide a source for it. I do not want this to turn into someone’s opinion about how they think it works. Thanks.
Last edited by Jbritt; 02-17-2010 at 11:34 AM. Reason: Wanted to address the pump octane levels
#3
Thank you.
I'd like to add that octane does not tell you anything about the speed of flame front propagation of the given fuel.
I'll dig up a paper later that I think you'll enjoy if you haven't spotted it linked from me before. It concerns tests done using 1 cylinder "diesel" motor set up for spark ignition and using various blends of alcohol and gasoline from e-10 through e-85 actually being more efficient and making more power than the "normal" compression gasoline engines using the same blends and straight gasoline. somewhere around e-40 i think was the most efficient/powerful.
there was also articles recently about someone pushing these types of engines for real world use.
I'd like to add that octane does not tell you anything about the speed of flame front propagation of the given fuel.
I'll dig up a paper later that I think you'll enjoy if you haven't spotted it linked from me before. It concerns tests done using 1 cylinder "diesel" motor set up for spark ignition and using various blends of alcohol and gasoline from e-10 through e-85 actually being more efficient and making more power than the "normal" compression gasoline engines using the same blends and straight gasoline. somewhere around e-40 i think was the most efficient/powerful.
there was also articles recently about someone pushing these types of engines for real world use.
#4
Thank you.
I'd like to add that octane does not tell you anything about the speed of flame front propagation of the given fuel.
I'll dig up a paper later that I think you'll enjoy if you haven't spotted it linked from me before. It concerns tests done using 1 cylinder "diesel" motor set up for spark ignition and using various blends of alcohol and gasoline from e-10 through e-85 actually being more efficient and making more power than the "normal" compression gasoline engines using the same blends and straight gasoline. somewhere around e-40 i think was the most efficient/powerful.
there was also articles recently about someone pushing these types of engines for real world use.
I'd like to add that octane does not tell you anything about the speed of flame front propagation of the given fuel.
I'll dig up a paper later that I think you'll enjoy if you haven't spotted it linked from me before. It concerns tests done using 1 cylinder "diesel" motor set up for spark ignition and using various blends of alcohol and gasoline from e-10 through e-85 actually being more efficient and making more power than the "normal" compression gasoline engines using the same blends and straight gasoline. somewhere around e-40 i think was the most efficient/powerful.
there was also articles recently about someone pushing these types of engines for real world use.
And if it breaks, I'v still got 48,000 miles of engine warrantee left!
#5
Thread Starter
The Black Pearl
iTrader: (2)
Joined: Jan 2009
Posts: 212
Likes: 0
From: Houston, Texas
Thank you.
I'd like to add that octane does not tell you anything about the speed of flame front propagation of the given fuel.
I'll dig up a paper later that I think you'll enjoy if you haven't spotted it linked from me before. It concerns tests done using 1 cylinder "diesel" motor set up for spark ignition and using various blends of alcohol and gasoline from e-10 through e-85 actually being more efficient and making more power than the "normal" compression gasoline engines using the same blends and straight gasoline. somewhere around e-40 i think was the most efficient/powerful.
there was also articles recently about someone pushing these types of engines for real world use.
I'd like to add that octane does not tell you anything about the speed of flame front propagation of the given fuel.
I'll dig up a paper later that I think you'll enjoy if you haven't spotted it linked from me before. It concerns tests done using 1 cylinder "diesel" motor set up for spark ignition and using various blends of alcohol and gasoline from e-10 through e-85 actually being more efficient and making more power than the "normal" compression gasoline engines using the same blends and straight gasoline. somewhere around e-40 i think was the most efficient/powerful.
there was also articles recently about someone pushing these types of engines for real world use.
As far as flame speed, you are completely correct. The speed of combustion with hydrogen is just a fun fact I enjoy sharing. Hydrogen is one of those gases that is fun to play with in a controlled environment. Just stay away from unintentional open flames or you lab will end up looking like the Hindenburg minus the thermite reaction.

#6
here's the epa paper http://www.epa.gov/otaq/presentation...-isaf-no55.pdf
and here is an article detailing Ricardo's demo program basically based on the findings in that EPA report.
http://www.pddnet.com/news-ricardo-a...engine-012710/
and Ford's "Bobcat" engine which implements the idea slightly differently but to the same effect- boost compression level and spraying e-85
http://www.autoblog.com/2010/01/14/f...ased-on-new-5/
this is a bit away from the gist of your OP so i might split this off later to keep the thread clean.
and here is an article detailing Ricardo's demo program basically based on the findings in that EPA report.
http://www.pddnet.com/news-ricardo-a...engine-012710/
and Ford's "Bobcat" engine which implements the idea slightly differently but to the same effect- boost compression level and spraying e-85

http://www.autoblog.com/2010/01/14/f...ased-on-new-5/
this is a bit away from the gist of your OP so i might split this off later to keep the thread clean.
#7
I have been linking to this Gasoline FAQ by Bruce Hamilton for years around here when ever octane comes up. I figure attaching a PDF of it here will make it easier to find in the future and goes well with this thread 
It's a good in depth read on gasoline and in section 6 explains octane.

It's a good in depth read on gasoline and in section 6 explains octane.
Last edited by zoom44; 07-27-2011 at 07:48 PM.
#9
#10
I have heard that too much octance can lead to extra carbon build up. I run 91 octane/no ethanol fuel and also did this in a 1.8T i used to have. i was able to monitor the knock sensor in that engine and found that in winter i didn't need to run premium. I assume that would be true with the rotary too, but people have told me I can't trust the knock sensor to tell me...
#11
Thread Starter
The Black Pearl
iTrader: (2)
Joined: Jan 2009
Posts: 212
Likes: 0
From: Houston, Texas
air density is always a concern when considering how fuel affects an engine. Cold air = good.
higher elevation will also allow you to run a lower octane gas. at about 5000+ feet, the octane requirements for an engine will decrease due to the air portion per combustion being lower. Less "air" at 5000 feet than at sea level.
higher elevation will also allow you to run a lower octane gas. at about 5000+ feet, the octane requirements for an engine will decrease due to the air portion per combustion being lower. Less "air" at 5000 feet than at sea level.
#12
In a normally aspirated motor, the octane requirement (for gasoline) will drop a little less than one point for every 1000 feet you gain in altitude.
But it is because of decreased compression, not "less air".
This does not apply to turbocharged vehicles since they make their own atmosphere.
#13
That's a misunderstanding based on the idea that higher octane burns at a different rate. One of the myths hopefully put to rest with threads like this.
There is no difference in deposits between octane levels- provided the same detergents are used by the fuel maker for each octane. you can't tell the quality of the gas by the octane level.
There is no difference in deposits between octane levels- provided the same detergents are used by the fuel maker for each octane. you can't tell the quality of the gas by the octane level.
#15
Thread Starter
The Black Pearl
iTrader: (2)
Joined: Jan 2009
Posts: 212
Likes: 0
From: Houston, Texas
Uh, not exactly.
In a normally aspirated motor, the octane requirement (for gasoline) will drop a little less than one point for every 1000 feet you gain in altitude.
But it is because of decreased compression, not "less air".
This does not apply to turbocharged vehicles since they make their own atmosphere.
In a normally aspirated motor, the octane requirement (for gasoline) will drop a little less than one point for every 1000 feet you gain in altitude.
But it is because of decreased compression, not "less air".
This does not apply to turbocharged vehicles since they make their own atmosphere.
 Didn't want to get into the changes of bring ambient pressure below 1 atm of STP. But, MM you are correct, it is not the actual air that is changing, but the lower air density affecting atmospheric pressure which in turn decreases the compression during the combustion event. Good stuff. Thread is becoming very informative, thanks for all the additions guys.
Didn't want to get into the changes of bring ambient pressure below 1 atm of STP. But, MM you are correct, it is not the actual air that is changing, but the lower air density affecting atmospheric pressure which in turn decreases the compression during the combustion event. Good stuff. Thread is becoming very informative, thanks for all the additions guys.
#16
isnt there more energy stored in octane than lets say, heptane? since it does have 1 more hydrogen. all you said makes sense...I once had a chemistry teacher try to explain that all 3 grades are the same crap unless you drive a race car, didn't quite understand him back then and dont remember what he said.
#18
#19
#20
Retard fail, see my response about compression ratio/seal effectiveness
ps: I'm mad as he'll and not going to take retarded BS any more. If you don't like it go F yourself. Yeah, it's that simple.
.
ps: I'm mad as he'll and not going to take retarded BS any more. If you don't like it go F yourself. Yeah, it's that simple.
.
Last edited by TeamRX8; 03-28-2010 at 03:46 AM.
#25
isnt there more energy stored in octane than lets say, heptane? since it does have 1 more hydrogen. all you said makes sense...I once had a chemistry teacher try to explain that all 3 grades are the same crap unless you drive a race car, didn't quite understand him back then and dont remember what he said.
Check out 2-methyl heptane:-
Code:
H H H H H H H | | | | | | | H-C-C-C-C-C-C-C-H | | | | | | | H C H H H H H /|\ H H H


